
The I atoms are much larger than the H atoms in CH 2I 2 and the H-H angle is smaller than the ideal 109 deg while the I-I angle is larger. In tetra-atomic boron trihydride (BH3), the dipole moment is zero, but that of ammonia. trigonal planar, trigonal pyramidal, bent B. Lone pairs > triple bond > double bond > single bondĪlso, atoms that are bonded to a central atom make a difference. Ch3br electron geometry The molecular geometry of BeF2 is linear. For each of the following species SeO3, BrO4, and ClF2+ provide the predicted molecular geometries, choosing from the options below-mentioned: A. The true bond angles will usually be distorted from the idealized angles in the pictures above because all bonds and non-bonding electron pairs don't have the same "size". Borane has 3 electron pairs and must be trigonal.īoth bonding and non-bonding electron pairs determine the structure but we name the geometry of molecules according to the arrangement of atoms.Įlectron Pairs 0 lone pairs 1 lone pair 2 lone pairs 3 lone pairs Only three of those pairs are bonded to another atom in ammonia. Methane and ammonia both have 4 electron pairs, arranged in a tetrahedron. When there are 6 pairs of electrons, they occupy the vertices of an octahedron (90 degree angles). The electron pair geometry is the arrangement of the (usually) pairs of bonding and non-bonding electrons around the central atom of the neutral molecule. Trigonal bipyramidal is the lowest energy, but the square pyramidal structure is pretty close and is also important. When there are 5 pairs of electrons, there are two possible arrangements: trigonal bipyramidal (90 and 120 degree angles) and square pyramidal (90 degree angles). The shape of the orbitals is planar triangular.

Four pairs will be arranged in a tetrahedron, 109 degrees apart. NOTES: This molecule is made up of 3 equally spaced sp 2 hybrid orbitals arranged at 120 o angles. Three pairs will be 120 degrees apart in a trigonal arrangement. Two pairs will always be 180 degrees apart, in a linear arrangement.
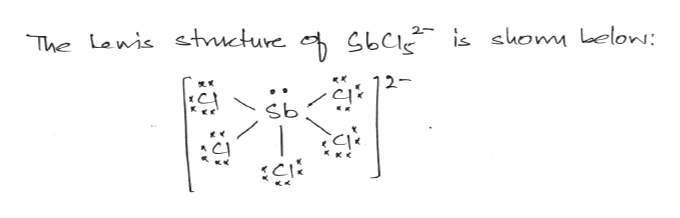
The electron pairs on the central atom will be arranged in such a way as to maximize their distance to the others. Let's look at a few examples: CH 4, NH 3, BH 3

The first step is to construct the best Lewis structure of the molecule. If a central atom (A) is surrounded by different atoms (B and C) in the molecule AB xC y, the relative sizes of B and C can affect the structure of the molecule.Single bonds are smaller than double bonds and double bonds are smaller than triple bonds.Bonding pairs are smaller than lone pairs because there are 2 positively charged nuclei pulling them in.All pairs of electrons, both bonding pairs and lone pairs, are important in determining the shape of a molecule.The idea behind this is that electrons in filled orbitals will repel each other because they have the same charge (just as magnets with the same polarity repel). We use Lewis structures along with Valence Shell Electron Pair Repulsion Theory to predict the structures of molecules.


 0 kommentar(er)
0 kommentar(er)
